- What is a BCI and How Does It Work?
- BCIs Enter the Real World
- Neuralink
- Synchron
- Blackrock Neurotech
- Paradromics
- Precision Neuroscience
- Market Outlook
- Obstacles and Challenges
- Looking Ahead: A New Breed of Neurotech Talent
Dr. Phil Kennedy stares at the ceiling of a Belizean operating room, determined and fearful. It’s 2014, and the pioneering neurologist – celebrated as the “father of the cyborg” for giving a locked-in patient the power to move a computer cursor by thought – has resorted to extreme measures. U.S. regulators had halted his research into decoding speech from neural activity, leaving him no test subjects. So, after 29 years of work, Dr. Kennedy took a risky leap: he paid $25,000 to have electrodes implanted in his own brain.
The 11.5-hour surgery (one no American hospital would perform) nearly stole his voice, as post-op complications left him temporarily mute. Yet when he recovered, he pressed on with experiments. As he silently mouthed words, signals from 65 neurons in his motor speech cortex flashed the same patterns as when he actually spoke aloud – proof that imagined speech could be captured and decoded. Though Kennedy’s self-implanted device had to be removed after a month (his skull incision refused to heal) and the daring self-experiment yielded a lot of criticism, it also became a proof of concept, promising technology that could give voice to the voiceless.
As of mid-2025, brain-computer interfaces (BCIs) are transitioning from science fiction curiosities and lab experiments into the neurotechnology industry.
What is a BCI and How Does It Work?
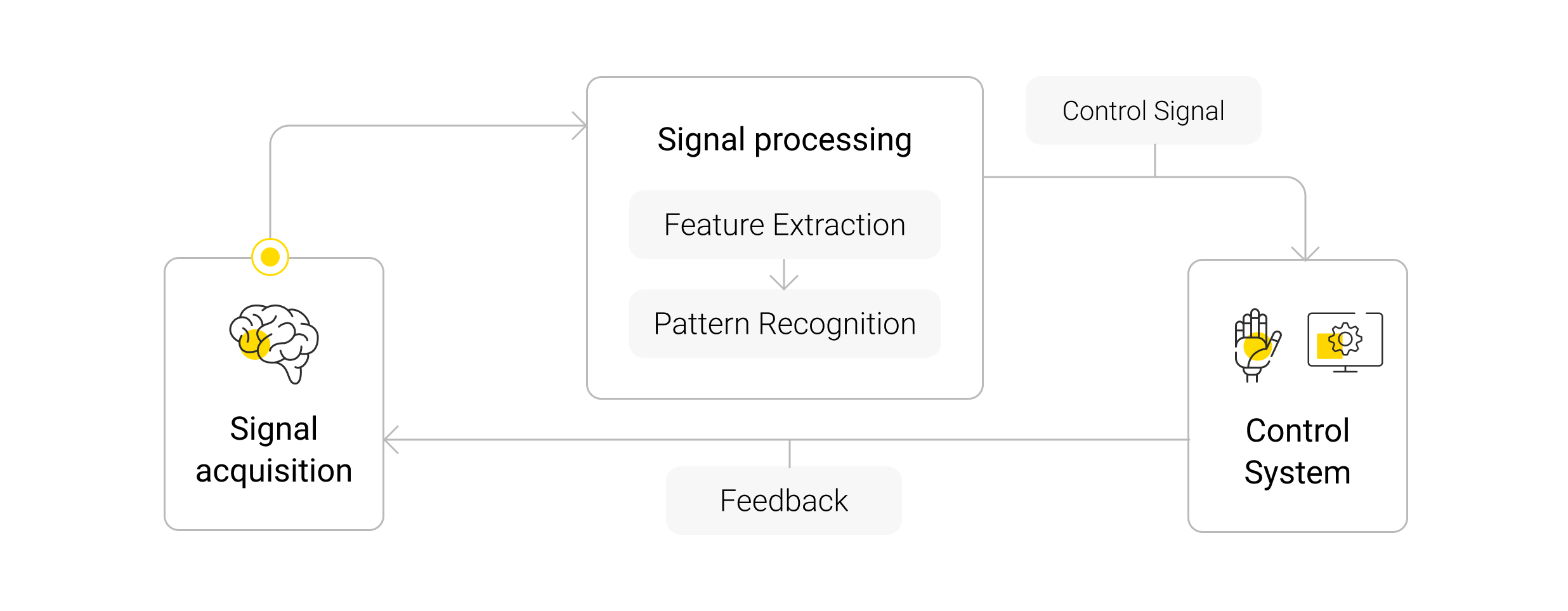
At its core, a brain–computer interface is a system that measures brain activity and converts it in real time into functionally useful outputs, changing the ongoing interactions between the brain and its external or internal environments. In plainer terms, a BCI translates thought into action. These systems come in many forms – from wearable headsets to surgically implanted microchips – but all share a similar pipeline . First is signal acquisition: electrodes or sensors pick up neural activity (electrical firing of neurons or field potentials in the brain). The signals may be captured noninvasively (e.g. EEG electrodes on the scalp) or via arrays of microelectrodes with varying degrees of invasiveness for higher fidelity. Next, the data goes through processing and decoding: algorithms filter noise and interpret the user’s intent from brainwave patterns. Essentially, the system must discern what action or message the brain is trying to convey – whether it’s moving a cursor or mentally “speaking” a word. Then comes the output: the decoded intent is translated into a command to control some external device or software, such as moving a robotic limb, controlling a wheelchair, or generating synthetic speech. Finally, most BCIs include a feedback loop, so the user can see or hear the result and adjust their mental strategy accordingly.
This closed-loop design – acquire, decode, execute, feedback – is the backbone of current BCI research.
Picture 1. Brain-computer interface schema
Today, the convergence of deep learning with neural data is yielding quite accurate decoders – e.g. speech BCIs infer words from complex brain activity at 99% accuracy and <0.25 second latency . Such feats were unthinkable ten years ago – in 2014, after his self-experiment, Dr. Kennedy was only able to produce about 290 short words. This illustrates how rapidly the technology to interpret brain signals (thanks to advances in AI and electrode design) has accelerated. Still, these remain experimental systems, often bulky and confined to labs. To gauge how close BCIs are to real-world deployment, we must survey the current players and clinical progress.
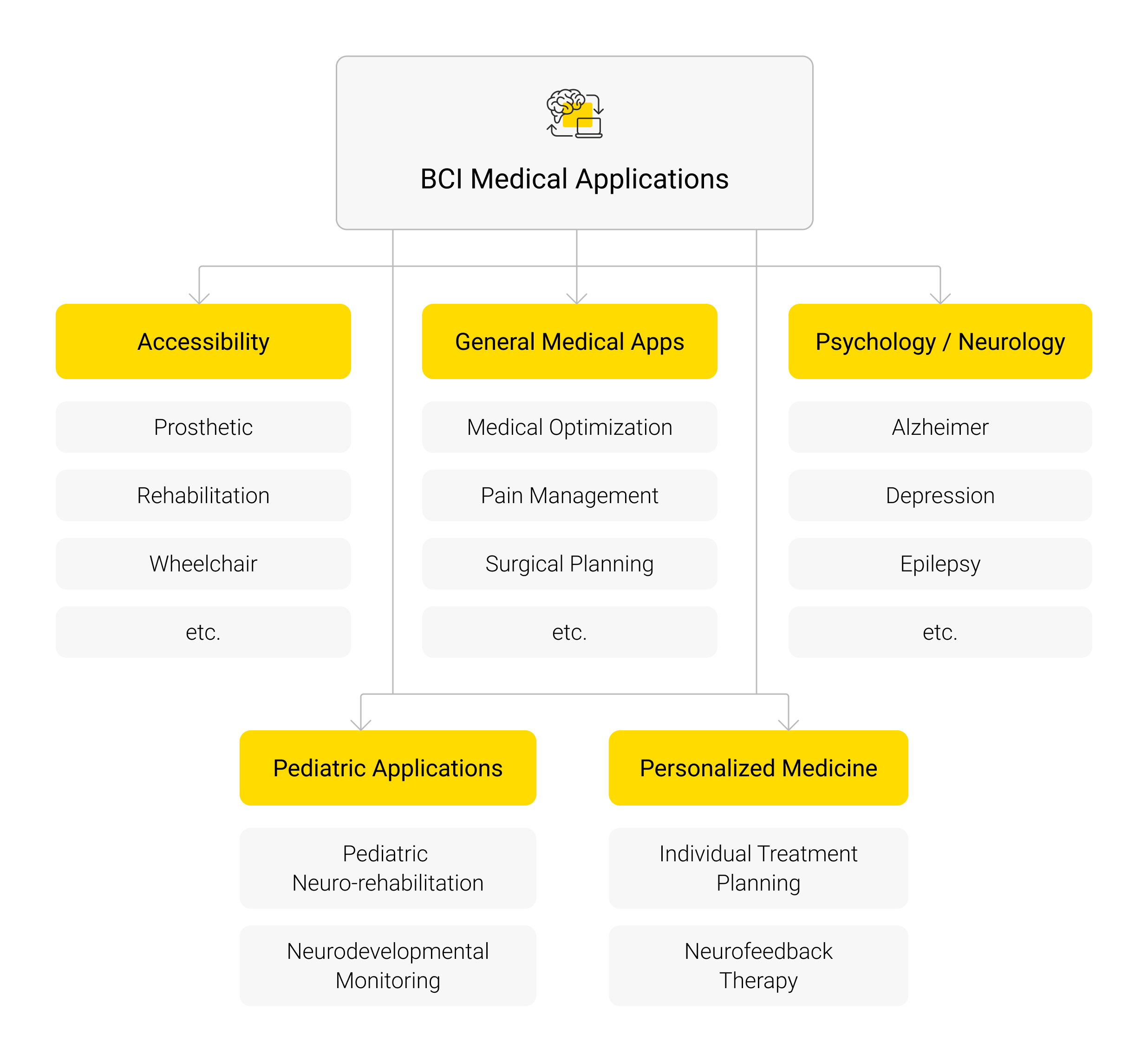
Picture 2. BCI medical applications
BCIs Enter the Real World
A flurry of neurotech startups and research groups are now translating BCI prototypes into clinical trials, with an eye toward medical commercialization. In the United States – home to many of the field’s pioneers – several venture-backed companies have moved to the forefront:
Neuralink
Perhaps the most publicized BCI company, Neuralink is developing an ultra-high-bandwidth implantable chip with thousands of micro-electrodes threaded into the cortex by a robotic surgeon. The coin-sized implant, sealed in the skull, aims to record from more neurons than any prior device. After years of animal tests (famously, a 2021 demo showed a monkey playing the video game “Pong” with its mind), Neuralink received FDA clearance in 2023 to begin human trials. By June 2025, the company stated: “Five individuals with severe paralysis are now using Neuralink to control digital and physical devices with their thoughts.”
Synchron
In contrast to Neuralink’s open-brain surgery, Synchron’s approach is much less invasive: their flagship device, the Stentrode, is a BCI delivered via blood vessels. Inserted through a catheter into the jugular vein, it is lodged in the motor cortex’s draining vein (the superior sagittal sinus) where it records brain signals through the vessel wall. This endovascular method avoids drilling into the skull at all. Synchron has already tested it in humans: in a four-patient trial, the Stentrode allowed participants with paralysis to control a computer, including texting, using thought alone. After 12 months, none of the patients had serious adverse events or blood vessel blockages, and the device stayed in place. In 2025, the big leap isn’t an increase of patients but mainstream ecosystem hooks (in partnership with Apple and NVIDIA) and the move toward a pivotal trial that could make Stentrode the first commercially scalable implanted BCI.
Blackrock Neurotech
This company has supplied neural electrode arrays for academic research for years – notably the Utah array, a bed-of-nails style implant used in many BCI studies, though its classic Utah spikes can cause scarring over time. The company is developing a new electrode tech called Neuralace, a flexible lattice for less invasive cortical coverage. As of 2025, Blackrock and its research partners are expanding trials, including in-home tests where paralyzed users live with the BCI daily.
Paradromics
A quieter contender until recently, Paradromics specializes in high-channel-count implants for ultra-fast data transmission. Its approach, called the Connexus BCI, uses a modular array with 421 electrodes and an integrated wireless transmitter. In June 2025, a University of Michigan team partnered with Paradromics to perform the first-in-human recording with the Connexus device. The BCI was temporarily implanted in a patient undergoing epilepsy surgery. The company plans to launch a full clinical trial by late 2025 after regulatory approval, with an initial focus on restoring speech for people who cannot talk – a natural target given the bandwidth of their implant. Paradromics’ CTO, Dr. J. R. Ryu, noted that the procedure showed the device “can be safely implanted using surgical techniques familiar to neurosurgeons anywhere,” paving the way for broader adoption if approved .
Precision Neuroscience
Co-founded by a Neuralink alumnus, Precision is developing a novel ultra-thin electrode array designed to slip between the skull and the brain with a minimally invasive approach. Their flexible chip, sometimes likened to a “brain film”, can be inserted through a slit in the dura (the brain’s protective lining) and conform to the cortical surface. This concept – a less invasive ECoG (electrocorticography) implant – aims to capture high-resolution signals without piercing brain tissue. In April of 2025, Precision’s device, codenamed Layer 7, received 510(k) clearance from the FDA; cortical interface is now authorized for commercial use with implantation durations of up to 30 days. The focus is on medical applications like enabling communication for patients with ALS. If it works, the approach could offer a compromise between noninvasive ease and invasive signal quality: essentially a “peel and stick” BCI that a neurosurgeon could install in under an hour.
The clinical trials underway span North America, Europe, Asia, and Australia, and involve an unprecedented number of human participants. By June 2025, we found around 90 active BCI trials testing implants for typing, mobility, stroke rehab, etc.
Notably, no BCI has yet been approved for general medical use – all these devices are experimental. But the field’s momentum is undeniable. In short, BCIs in 2025 stand roughly where gene therapies did in the 2010s or heart stents in the 1980s: on the cusp of graduating from experimental status to regulated clinical use, driven by a mix of startup innovation, academic research, and patient demand.
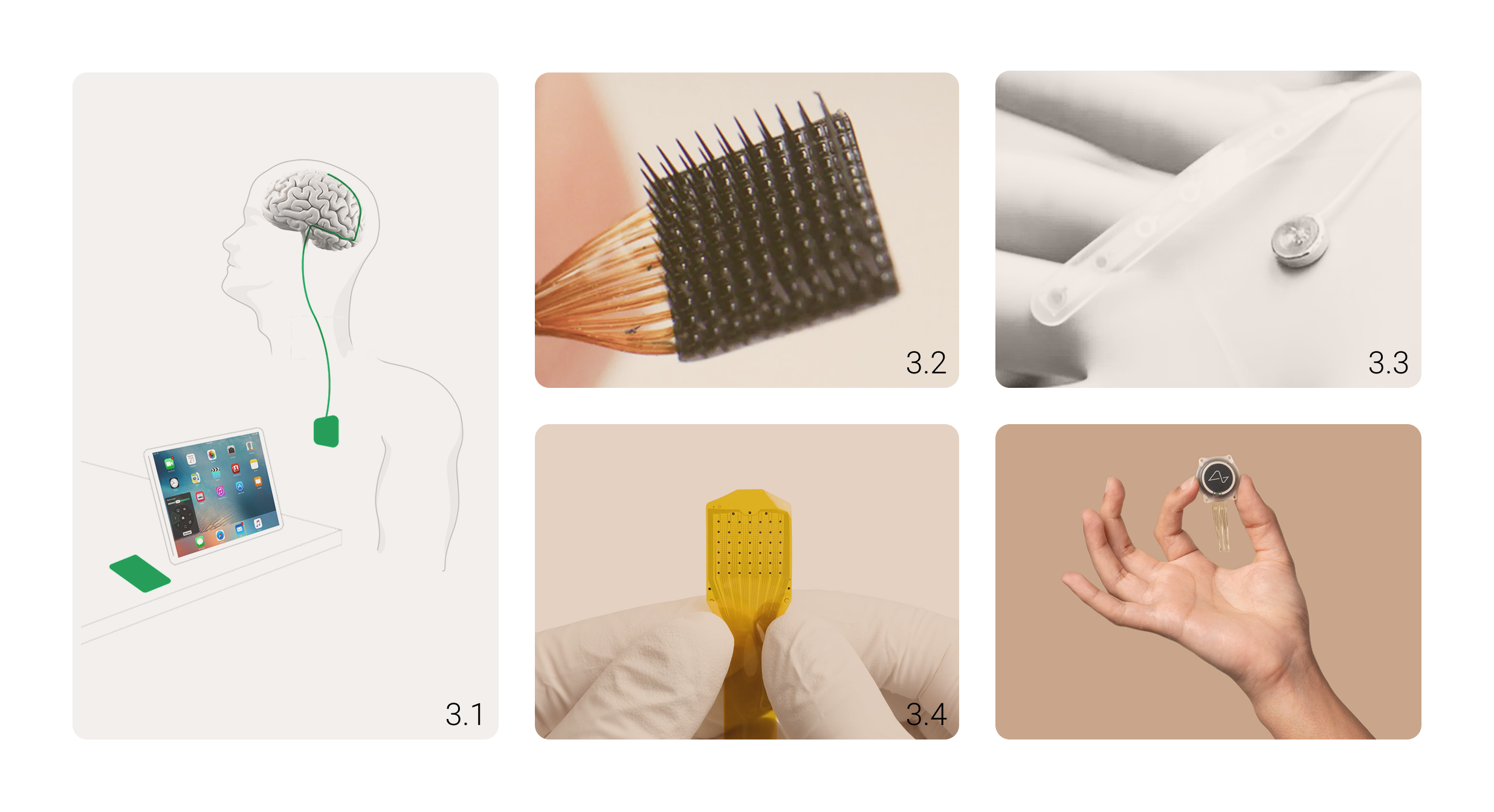
Picture 3. 3.1 BCI from Synchron (Source)
3.2 BCI from Blackrock Neurotech (Source)
3.3 BCI from Paradromics (Source)
3.4 BCI from Precision Neuroscience (Source)
3.5 BCI from Neuralink (Source)
Market Outlook
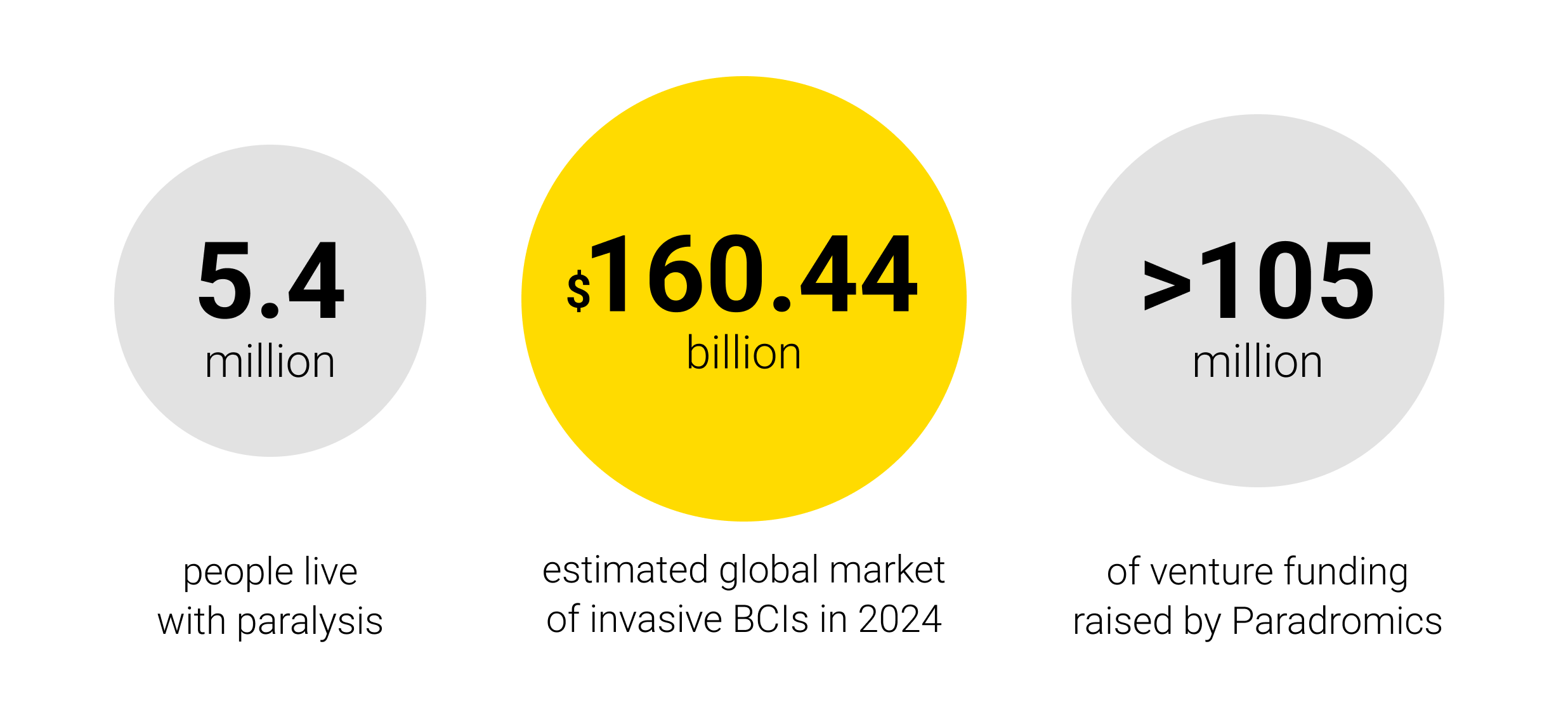
The addressable market for BCIs in healthcare is significant. In the United States alone, an estimated 5.4 million people live with paralysis that impairs their ability to use computers or communicate. Even if only a fraction of these individuals ultimately qualify or opt for neural implants, the life-changing impact per patient could be enormous – for example, giving a completely locked-in patient the ability to text, or a quadriplegic person the ability to steer their wheelchair by thought. While current sales are essentially zero (with devices still in trials), projections for the BCI industry’s growth are strikingly high. Private market studies compiled by the U.S. GAO estimate the global BCI market will expand by 10–17% annually until 2030. Grand View Research estimates the global market of invasive BCIs at $160.44 billion in 2024, driven initially by uses in paralysis, rehabilitation, and prosthetics. These rosy forecasts reflect heavy investment: in the past five years, venture capital and corporate funding for neurotech startups have surged. For example, Neuralink has reportedly raised over $650 million to date, and Paradromics has secured more than $105 million of venture funding, in addition to $18 million from NIH and DARPA grants as of February 2025.
Beyond healthcare, tech giants and other industries are eyeing BCIs for wider applications – a fact that swells the market hype (if not the near-term reality). Companies are investigating BCIs for the workplace (e.g. hands-free computing or monitoring employee fatigue), for gaming and entertainment, and even for national defense. Facebook (Meta) had an internal BCI research program aiming for AR/VR interfaces (though it pivoted to noninvasive methods), and the U.S. military’s DARPA has funded projects on “telepathic” soldier communication. While many non-medical BCI concepts remain speculative or face ethical issues, the buzz around potential mass-market BCIs has drawn more capital and talent into the field, further accelerating progress.
The coming two or three years will be pivotal: early trial results will either validate the hopes or temper the hype. If one company achieves FDA approval, it could open the floodgates for investment and broader adoption – much as the first gene therapy approval did for biotech. The global competition is also heating up, which brings us to how different regions are faring in this neurotech race.
Obstacles and Challenges
For all the excitement, BCIs in 2025 face a gauntlet of technical, regulatory, and practical challenges. The road from demonstration to widespread clinical adoption is not a smooth one. Invasiveness and safety remain the foremost hurdle: most high-resolution devices require drilling into the skull, risking infection or brain injury, while less invasive stentrodes still need vascular navigation. Durability and signal stability are also critical. Utah arrays, for instance, often lose signal from over 60% of their electrodes within one year as scar tissue muffles the signals.
Decoder generalization poses another barrier as today’s BCIs must be painstakingly trained to each user’s unique neural patterns, and performance can fluctuate daily with fatigue or mood. Regulatory uncertainty compounds these issues, as agencies grapple with evaluating devices that straddle medical treatment and cognitive augmentation, while also requiring plans for long-term maintenance if companies fail. Data privacy and security remain under-addressed: with wireless implants, brain signals could be intercepted or misused, raising the specter of “neurohacking.”Finally, there is the barrier of expectations vs. reality. Media hype (and some zealous CEOs) have promised a lot: from curing depression to letting you save and replay memories. The truth is that the first generation of BCIs will be limited – remarkable, yes, but not miracles or mind-control gadgets. If unrealistic expectations build and then are unmet, it could trigger a backlash or funding bust – a classic Gartner “hype cycle” crash. Indeed, experts caution about deceptive marketing, noting some companies might exaggerate benefits or minimize risks.
None of these challenges are insurmountable, but they demand careful engineering and policy. It’s a marathon, not a sprint, and we are perhaps at mile 13 – the midpoint where fatigue sets in but the finish line (of initial commercial viability) comes into view.
Looking Ahead: A New Breed of Neurotech Talent
As BCIs advance, one often overlooked need is for human capital, and the future of BCIs will be written by teams – teams of engineers, doctors, patients, and yes, business leaders and policymakers working in concert.
Clinicians provide insight into surgical feasibility and patient needs, while engineers optimize sensors and algorithms. Right now, there is a limited pool of people who are fluent in “brain” and “computer” in equal measure. Companies are scrambling to hire neural engineers – people who might rig a circuit board in the morning and analyze spike waveforms in the afternoon. Similarly, AI scientists with neuroscience knowledge are in high demand to design the decoding algorithms. On the clinical side, bringing BCIs to patients will require neurologists and rehabilitation specialists trained in this new tech, much as cardiac surgeons had to learn to implant stents decades ago.
The long-term vision for BCIs extends to integration with other technologies. Consider combining BCIs with AI assistants, such as your brain signals directly querying ChatGPT-2050, or with AR/VR goggles for immersive control. These integrations will call for even broader skill sets, including UI/UX designers who can craft user experiences for something as intimate as a brain interface. It’s a daunting but exhilarating prospect: we are effectively crafting a new human-machine language, and doing so demands pioneers fluent in both sides of the exchange.By mid-2025, the brain-computer interface field is at an inflection point. Dramatic stories – like a scientist implanting himself to push the research forward, or a patient speaking after years of silence through a neural decoder have transitioned BCIs from obscure lab projects to headlines and boardroom discussions. For healthtech CEOs and industry strategists, BCIs represent a frontier market that, while risky, aligns with powerful trends: aging populations needing assistive tech, leaps in AI that can interpret complex data, and a world increasingly comfortable with tech under the skin.